Organoids
Organoids are a kind of "micro-organ" formed by stem cells cultured in vitro. Organoids contain a variety of tissue-specific cell types and self-organize into three-dimensional tissue structures, which can partly mimic the structure and function of native organs. Due to the great application potential of organoid technology in biomedical translational research fields such as disease research, drug screening, drug toxicity toxicological response, gene and cell therapy, in 2019, the New England Journal of Medicine evaluated organoids as "Preclinical models of human disease".
"An organoid is a three-dimensional construct composed of multiple cell types that originates from stem cells by means of self-organization and is capable of simulating the architecture and functionality of native organs. Organoids permit in vivo and in vitro investigation and represent one of the latest innovations in the quest for a model to recapitulate the physiologic processes of whole organisms.”
The potential of organoids as in vitro systems to culture human epithelial cells from almost any organ is discussed below. Organoids are 3D structures that mimic organ structure and function. They are generated from stem cells or tissue-specific progenitor cells and can be used in both basic and translational research. The limitations of organoids are highlighted, such as the trade-off between optimal conditions for each contained cell type and the need to further optimize co-culture conditions. The use of tumor-derived organoids to mimic the effects of novel cancer immunotherapies is also mentioned. Overall, organoids provide a promising tool for the study of human organ development, disease, and treatment.

A number of model systems have been developed over the years for the study of epithelial cells and their interaction with the immune system. On the feeder cell layer, the method of culturing epidermal stem cells has been used for more than 50 years. Recent advances have made it possible to transform long-term cultures of adult intestinal stem cells into liver, skin epidermis, and other epithelial cells in a manner that closely mimics in vivo conditions and allows disease modeling in vitro. In the basic approach, adult stem cells are embedded in an extracellular matrix-rich (ECM) hydrogel supplemented with growth factors. This results in the proliferation of epithelial stem cells into 3D cell populations, consistent with the generation of differentiated cell types. These near-native clusters of epithelial cells are called "organoids," and it is also possible to generate stem cell-rich organoids, or epithelial and stromal cells, using sheets of tissue composed only of epithelial cells. Organoids can also be generated from pluripotent stem cells (PSC), either embryonic stem cells or induced pluripotent stem cells (iPSC). Organoids are defined as 3D clusters of cells that grow from (pluripotent or adult) stem cells and spontaneously organize into organoid or tissue-like structures that are rich in cell types normally present in the tissues of origin. "Their associated organoids represent organs in which epithelial cells have dominant functions, such as the kidney, stomach, and intestine. In contrast to normal cell culture, organoid techniques typically require long-term proliferation and differentiation of stem cells under specific conditions (e.g., serum-free culture) that ensure that organoid cultures are not subject to the lot-by-batch variations that typically occur with serum-based cultures. In addition, adult stem-cell-derived organoids contain many types of (epithelial) differentiated cells that are typically present in the tissue of origin, and they are genetically stable and retain an autogenic epithelial phenotype, resulting in cellular heterogeneity as compared with generally homogeneous common cell-based cultures. These features of organoids not only allow a wide range of experimental applications, such as imaging, molecular analysis, and gene editing, but also enable a reductionistic approach to the study of epithelial-immune cell interactions. With the development of organoid models for cancer and other immunogenic diseases, such as inflammation and intestinal diseases (IBD), the role of immune system components in the pathogenesis of epithelial tissue diseases can be further explored.

Adult stem cell-derived organoid cultures are typically established by embedding isolated adult stem cells (when defined) or single-cell suspensions of the desired organ in extracellular matrix (ECM) hydrogels. In the case of intestinal organoids, crypt cultures isolated from the small intestine or colon are sufficient to grow organoids, although organoids can also be grown from isolated (flow-sorted) adult intestinal stem cells. Epithelial organoid media were based on media supplemented with growth factors relevant to the organ of interest.
For example, survival of mouse small intestinal organoid cultures in supplemented media using the growth factors noggin, R-spondin 1 or R-spondin 3 and epidermal growth factor (EGF) generally does not require the addition of exogenous WNT because Panes cells in the culture will produce sufficient WNT for organoid propagation. This is not the case for human small intestine or colon organoids or mouse colon organoids, where exogenous recruitment of WNT is essential for stem cell (and culture) maintenance. Thus, organoids from other tissues (e.g., airways, liver, pancreas, skin, and bladder) require supplementation of the culture with other growth factors. Importantly, the same applies to tumor-derived organoids. Tumor cells often lose their dependence on certain growth factors due to genomic mutations, so removal of these growth factors from the culture medium can be used to select cultures of pure tumor origin.
To study fetal development
Pluripotent stem cell (PSC) -derived organoids are generated by providing culture conditions that allow PSC to differentiate into the germ layer of the developing target tissue. To generate intestinal organoids from PSC, activin a was supplemented to stem cell medium to allow differentiation into endodermal progenitors, resulting in the formation of spheroids. These spheroids were subsequently cultured in ECM hydrogels and media containing the classical small intestinal organoid growth factors eGF, noggin, and Rspondin 1 or Rspondin 3. Trigger cells differentiate into intestinal epithelial and mesenchymal cells.

Interactions with epithelial cells that influence immune cell phenotypes

Mouse ISC subsets can function as nonclassical antigen-presenting cells in the MHCII class of molecules. Through antigen presentation, these ISC activate lamina propria resident CD4 T cells to produce various T cell-derived cytokines, which in turn influence epithelial differentiation. However, coculture of intestinal organoids with antigen-specific CD4 T cells did not appear to cause T cell activation, but T cells co-cultured with sorted ISC did. This difference could be explained by the relatively small number of ISC in organoid cultures, thereby reducing the sensitivity of this specific assay.

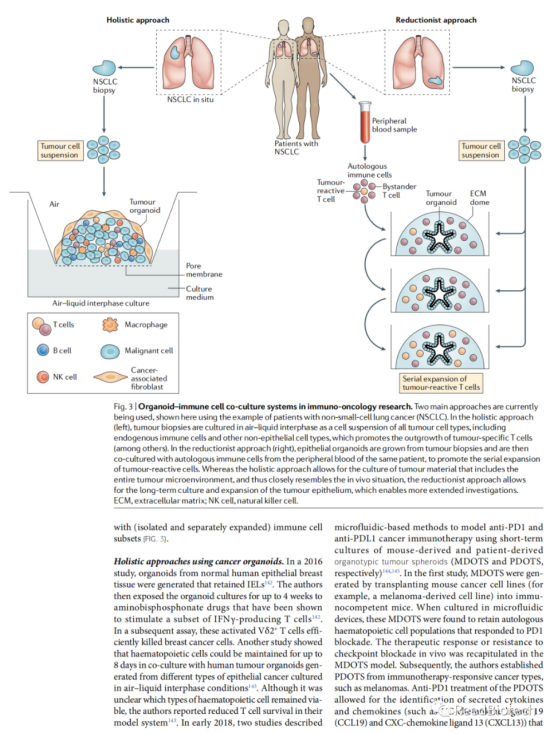
To study the tumor microenvironment
Summary and Future
Epithelial organoid cultures, whether derived from iPSCs or adult stem cells, provide a promising platform for immunological studies for a variety of applications. First, organoids allow reductive studies of the complex and intimate interactions between epithelial and immune cells. The influence of the immune system on epithelial differentiation and function has been extensively studied in the gut. It will be interesting to see if similar principles apply to other organoid systems, such as the skin or lung, which also interact with commensal microbes and immune cells. Epithelial composition changes at the time of infection and upon cytokine triggering. Thus, potential bidirectional interactions between the immune system and the epithelium, such as that seen in the gut, can be easily studied in vitro using organoids. These studies will increase our understanding of the biology of complex inflammatory diseases, from IBD to multiple sclerosis, psoriasis, and asthma.
Organoids have also been used to study host-microbe interactions. The next step in this direction would be to add immune system components to infected organoids. A limited number of methods using this triple coculture have been developed, mainly to model viral or bacterial pathogenic infections. Here, the organoids are already infected with pathogens (viral or bacterial) before being cocultured with immune cells. In recent years, it has been shown that commensal microbiota has a key role in protecting mammalian homeostasis, and it has been suggested that this is based at least in part on interactions with the epithelium. Epithelial organoid technology provides a unique opportunity to study all three components in the defining system: epithelium, immune system, and commensal microorganisms. The interaction between these components plays a crucial role in many human diseases, such as IBD and asthma. Therefore, a better understanding of these interactions and their consequences is of great interest. In the future, optimization of (triple) co-culture systems will be important to study these interactions under both homeostatic and pathological conditions and may lead to novel therapeutic approaches.
Finally, tumor-derived organoids, representing the transcriptional and mutational profile of the original tumor, provide novel reliable model systems for the interaction of the immune system with tumor cells. Several tumor-derived organoid systems have been shown to faithfully recapitulate tumor responses to various therapies, including chemotherapeutic agents and radiation therapy. This faithful generalization of in vitro tissue responses is of interest in other pathological conditions and is exemplified in the case of cystic fibrosis, where patient-derived organoids can be used to predict patient response to therapy. The use of organoids as a platform for developing and testing personalized medicine approaches is attractive because they are essentially cultures of primary patient material. Although this now has the potential to be used for cystic fibrosis and cancer, it may also be for inflammatory diseases affecting epithelial tissues. To this end, organoid based bioassays need to be standardized and clinically graded, and diagnostic tests that include organoids should be incorporated into clinical trials. As cancer immunotherapy plays an increasingly important role in the clinic, several reports have suggested that tumor-derived organoids can be used to mimic the effects of this new therapy. However, although tumor-derived organoids show promise for future research, some important limitations should be considered. Organoids are often derived from biopsies that represent only a small fraction of the tumor tissue. Therefore, the complexity of the overall tumor will always be underestimated by organoids. In addition, tumor-derived organoids are not exposed to external stresses that occur in situ, such as hypoxia or immune selection. Selection can affect tumor clone growth, leading to situations in which the dominant in vitro clone is less dominant in situ and vice versa. Importantly, these limitations become even more important when considering hypermutated tumors, such as MMR-deficient CRC or NSCLC. More generally, co-culture conditions for organoids are often a compromise between optimal conditions for each of the cell types included. Since culture conditions can affect cell behavior in a number of ways, it is important to keep this in mind when considering long-term co-culture experiments. To this end, one of the priorities for researchers in the coming years will be to further optimize the co-culture conditions, keeping in mind factors such as medium composition and the type of ECM used. Noting these limitations, organoids currently provide the most accurate in vitro system for human epithelial cell culture in almost any organ and show great promise for future basic and translational research.
