Programmed death-1 receptor (PD-1) and Programmed Death Ligand 1(PD-L1) are important immune checkpoint molecules and play a key role in immune regulation. As a member of the CD28 family, PD-1 (CD279) belongs to type I transmembrane proteins, and its structure is composed of 288 amino acids. Its ligand PD-L1 (CD274) is composed of 290 amino acids and has unique structural characteristics: The extracellular region contains IgV and IGC-like domains, which are anchored to the cell membrane through the transmembrane region and extend to a shorter intracellular end. After the two specifically combine, they trigger the immunosuppressive signaling pathway, maintain immune homeostasis and prevent excessive immune responses. Tumor cells hijack this physiological regulatory mechanism to form immune escape by abnormally high expression of PD-L1, thereby evading the anti-tumor immune response mediated by T cells.
Synthesis and Expression of PD-1 and PD-L1
PD-1: It is mainly expressed on the surface of activated T cells, B cells and NK cells, and its encoding gene is located on human chromosome 2.
PD-L1: It is widely distributed in tumor cells, immune cells (such as macrophages, dendritic cells) and non-immune cells (such as vascular endothelial cells). Studies have shown that inflammatory factors in the tumor microenvironment (such as IFN-γ) can significantly induce the expression of PD-L1.
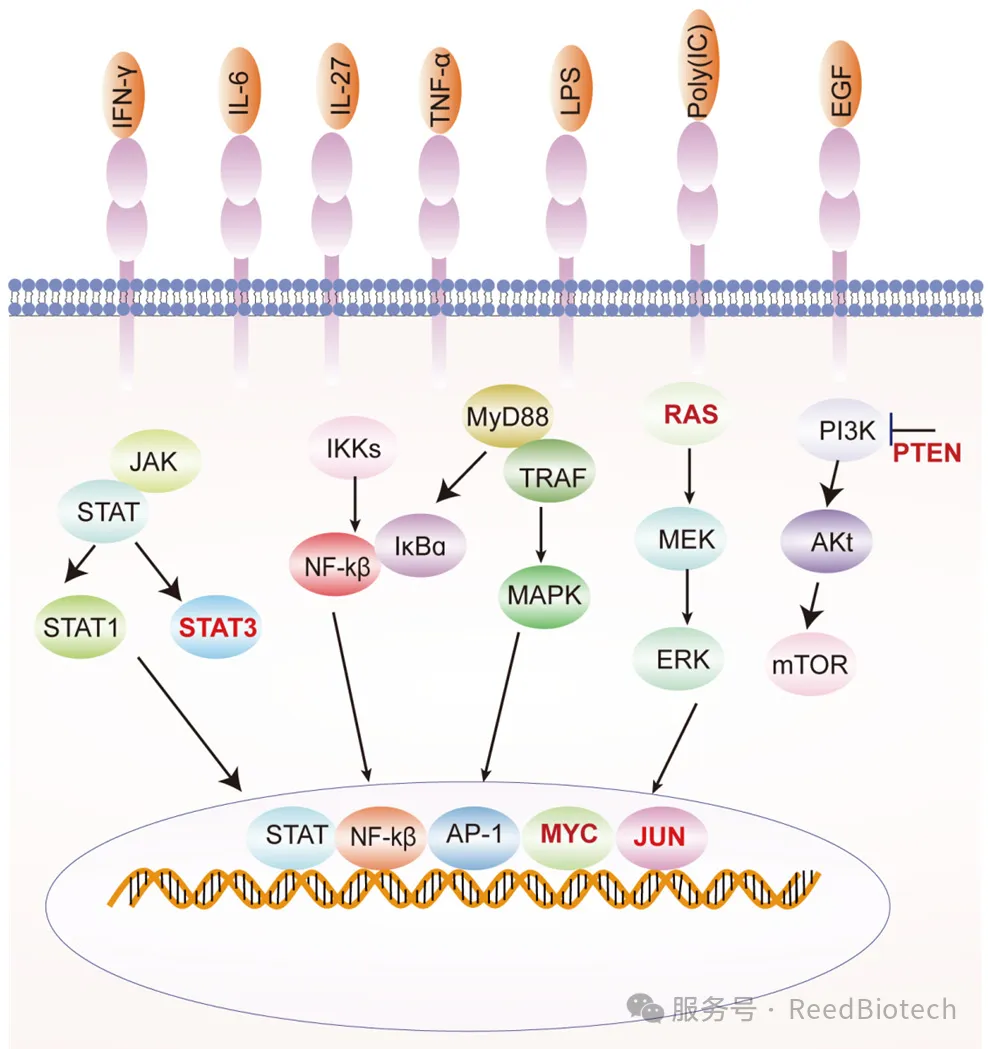
Classification of PD-L1 expression [5]
The mechanism of action of PD-1/PD-L1: The key pathway of immune escape
Inhibit the activity of T cells
When PD-1 binds to PD-L1, it triggers the signaling pathways within T cells (such as the activation of SHP-2 phosphatase), inhibits the secretion of cytokines such as IL-2 and IFN-γ, resulting in the obstruction of T cell proliferation and functional exhaustion.
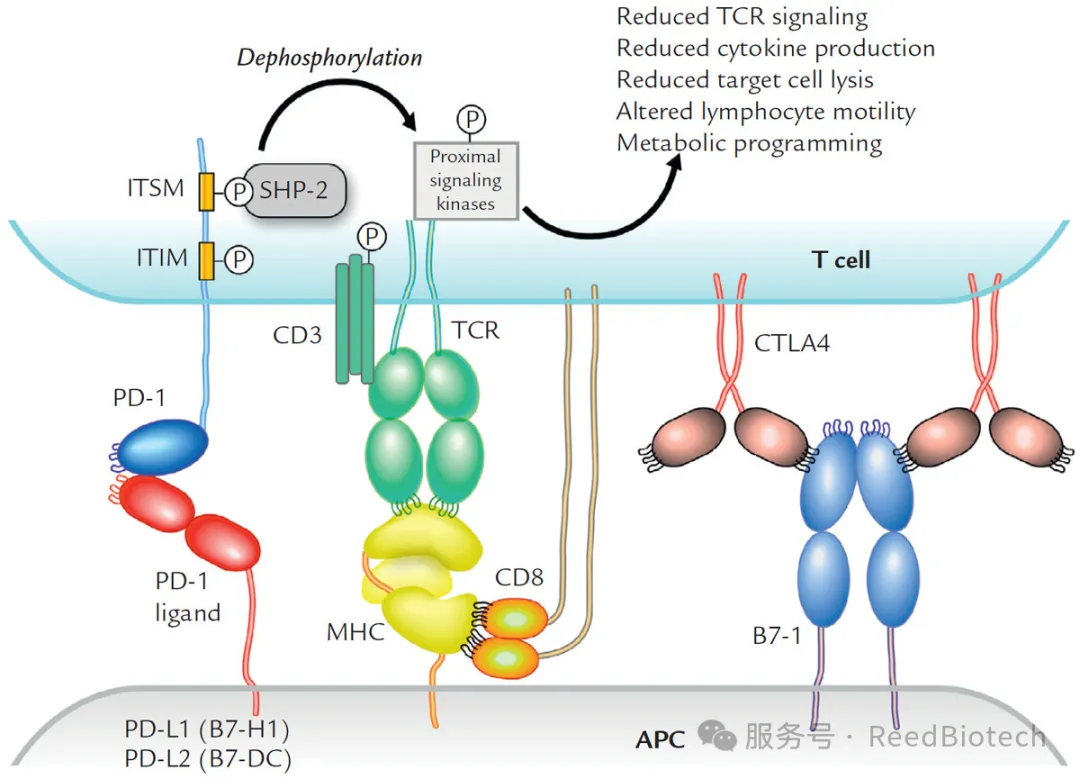
The interaction of PD-1 and PD-L1 reduces T-lymphocyte functionn[1]
The "accomplice" of tumor immune escape
Tumor cells bind to PD-1 on the surface of T cells through high expression of PD-L1, forming an "immune brake" and avoiding recognition and elimination [5]. For example: In melanoma patients, the response rate of PD-L1 positive tumors to PD-1 inhibitors is as high as 40% [1]. In various solid tumors such as lung cancer and kidney cancer, the expression level of PD-L1 is associated with poor prognosis [4].
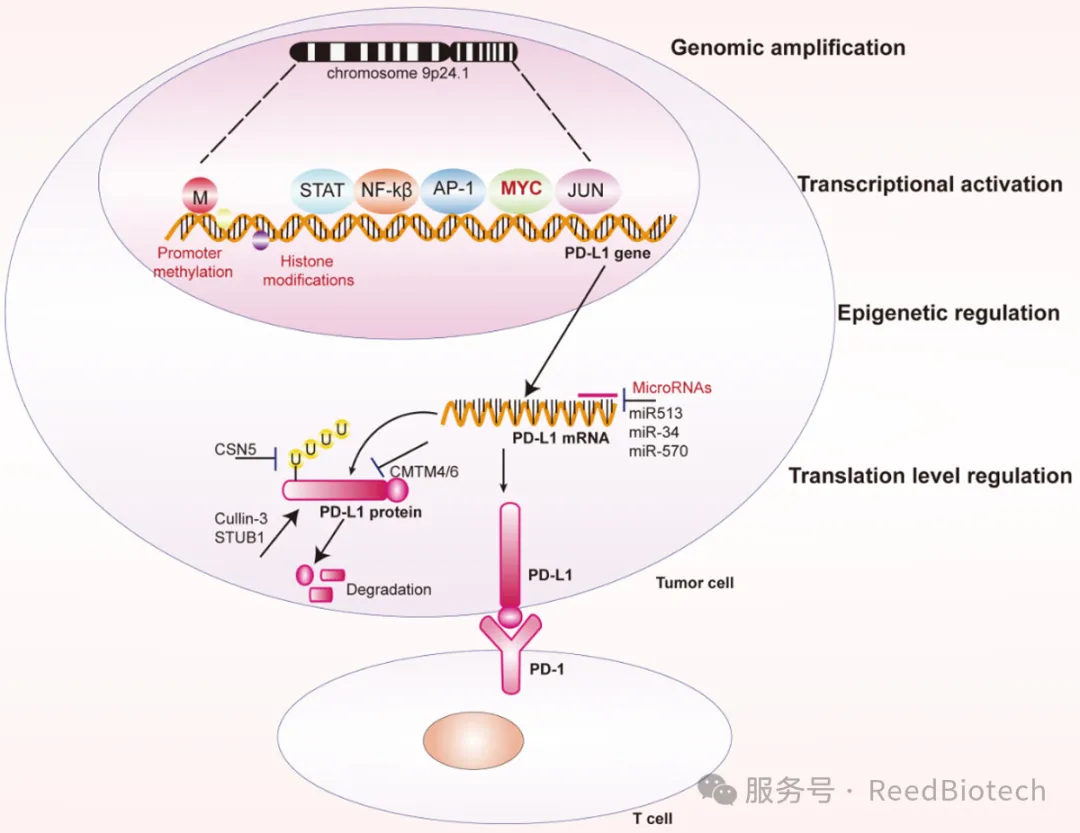
Regulation of PD-L1 expression in cancer cells at different levels.[2]
The physiological significance of PD-1/PD-L1: Balancing immunity and tolerance
Maintain autoimmune homeostasis
Under normal physiological conditions, the PD-1/PD-L1 signaling can prevent excessive immune responses and avoid tissue damage (such as autoimmune diseases). For example: PD-1 deficient mice are prone to autoimmune myocarditis [4]. In viral infections, the upregulation of PD-L1 can limit immunopathological damage [3].
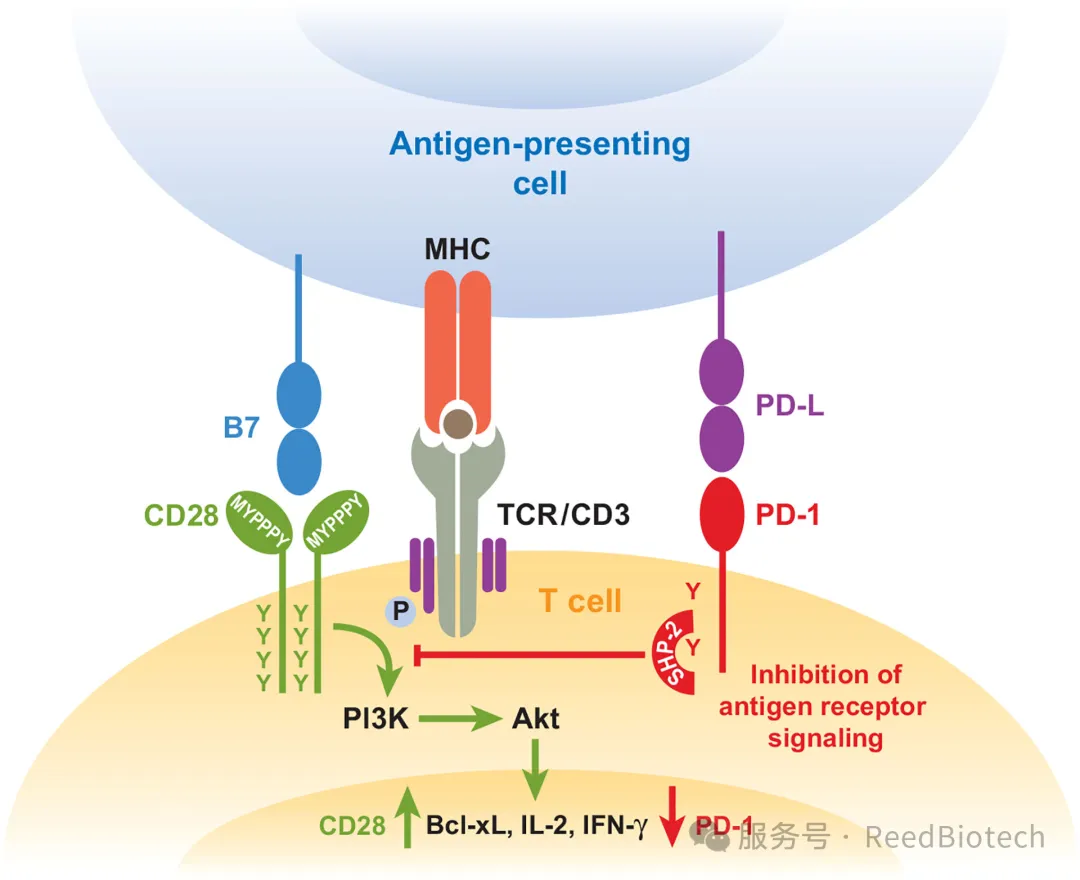
Ligation of PD-1 dampens TCR signaling but can be overcome by CD28 costimulation[3]
Complex Interplay Within the Tumor Microenvironment
PD-L1 not only inhibits anti-tumor immunity, but may also be involved in processes such as tumor angiogenesis and metabolic reprogramming, further promoting tumor progression [2][5].
Clinical Significance of PD-1/PD-L1 Detection
Guide on Immunotherapy
The expression level of PD-L1 has become an important biomarker for predicting the efficacy of PD-1/PD-L1 inhibitors. For example: In patients with high expression of PD-L1 in tumor tissues (≥50%), the objective response rate of pembrolizumab was significantly increased [1]. The dynamic monitoring of free PD-L1 in blood samples can reflect the therapeutic effect.
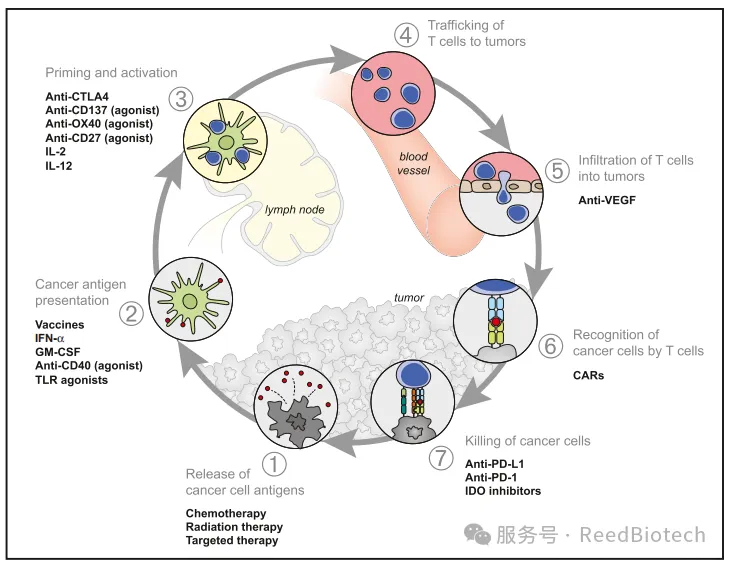
Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle[5]
Disease diagnosis and prognosis assessment
Prediction of tumor immunotherapy:
High serum sPD-L1 (soluble PD-L1) levels may be associated with increased tumor burden and immunosuppressive microenvironment, suggesting a poorer potential response to PD-1/PD-L1 inhibitors (such as pembrolizumab). Dynamic monitoring of sPD-1(soluble PD-1) changes can evaluate the treatment response: For example, the decrease of sPD-1 after treatment may be related to the recovery of T cell function.
Disease prognosis assessment:
In non-small cell lung cancer (NSCLC) and melanoma, elevated baseline sPD-L1 is associated with a shorter overall survival (OS). In chronic viral infections (such as HIV and HBV), an increase in sPD-1 indicates T cell exhaustion and an increased risk of disease progression.
Surveillance of autoimmune diseases:
The elevated serum PD-1 levels in patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) are positively correlated with disease activity (such as SLEDAI score), which may reflect excessive activation of the immune system.
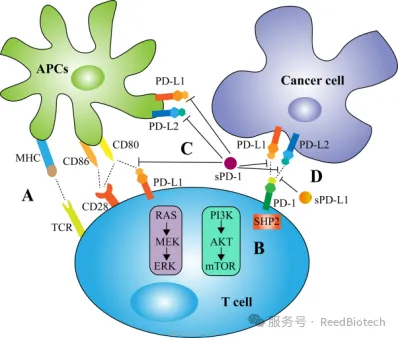
Biological activity of membrane and soluble PD-1/PD-L1 in tumor immunity.[6]
New Product Recommendations:
|
RE3054H |
Human PD-1 ELISA Kit |
|
RE3054M |
Mouse PD-1 ELISA Kit |
|
RE3055M |
Mouse PD-L1 ELISA Kit |
|
RE3055H |
Human PD-L1 ELISA Kit |
|
RE3054MF |
MF-Mouse PD-1 ELISA Kit(Micro Fast) |
|
RE3055MF |
MF-Mouse PD-L1ELISA Kit(Micro Fast) |
Cited References
[1]The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma
[2]Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy
[3]Tumor-associated B7-H1 promotes T-cell apoptosis: Apotential mechanism of immune evasion
[4]PD-1 and Its Ligands in Tolerance and Immunity
[5]Oncology Meets Immunology:The Cancer-Immunity Cycle
[6]Biological Characteristics and Clinical Significance of Soluble PD-1/PD-L1 and Exosomal PD-L1 in Cancer
[7]Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory
