Research Perspective | From Crystal Triggers to Cytokine Storms: Understanding the Core Inflammatory Axis of Acute Gout in One Read
I. Introduction
An acute gout flare can cause a joint to swell to the size of a “steamed bun” within 24 hours, bringing excruciating pain to patients. The culprit is urate crystals—hyperuricemia leads to the deposition of monosodium urate (MSU) crystals in joints, bursae, and soft tissues [1]. These crystals induce cellular stress and injury, triggering a severe inflammatory response. Rapid effusion in the joint cavity increases pressure on local nerves, resulting in intense pain.
During the inflammatory cascade of acute gout, multiple inflammatory mediators are released, reinforcing and amplifying each other—particularly cytokines IL-1β and TNF-α.
In the past, research spotlights have mostly focused on IL-1β, while overlooking its “longstanding partner,” TNF-α. Over the past five years, high-throughput proteomics and single-cell sequencing have confirmed that TNF-α not only “ignites the fire” but also acts as an “amplifier” sustaining IL-1β release. How do these two cytokines cross-talk? Can clinical interventions block both targets simultaneously? This article unpacks the latest findings for you.
II. Mechanism at a Glance: From MSU Crystals to the “TNF-α ➜ IL-1β” Positive Feedback Loop
① Initiation signal
Phagocytosis of monosodium urate (MSU) crystals by macrophages → Instant activation of the TLR4/NF-κB pathway → Transcriptional upregulation of both pro-IL-1β and TNF-α [2].
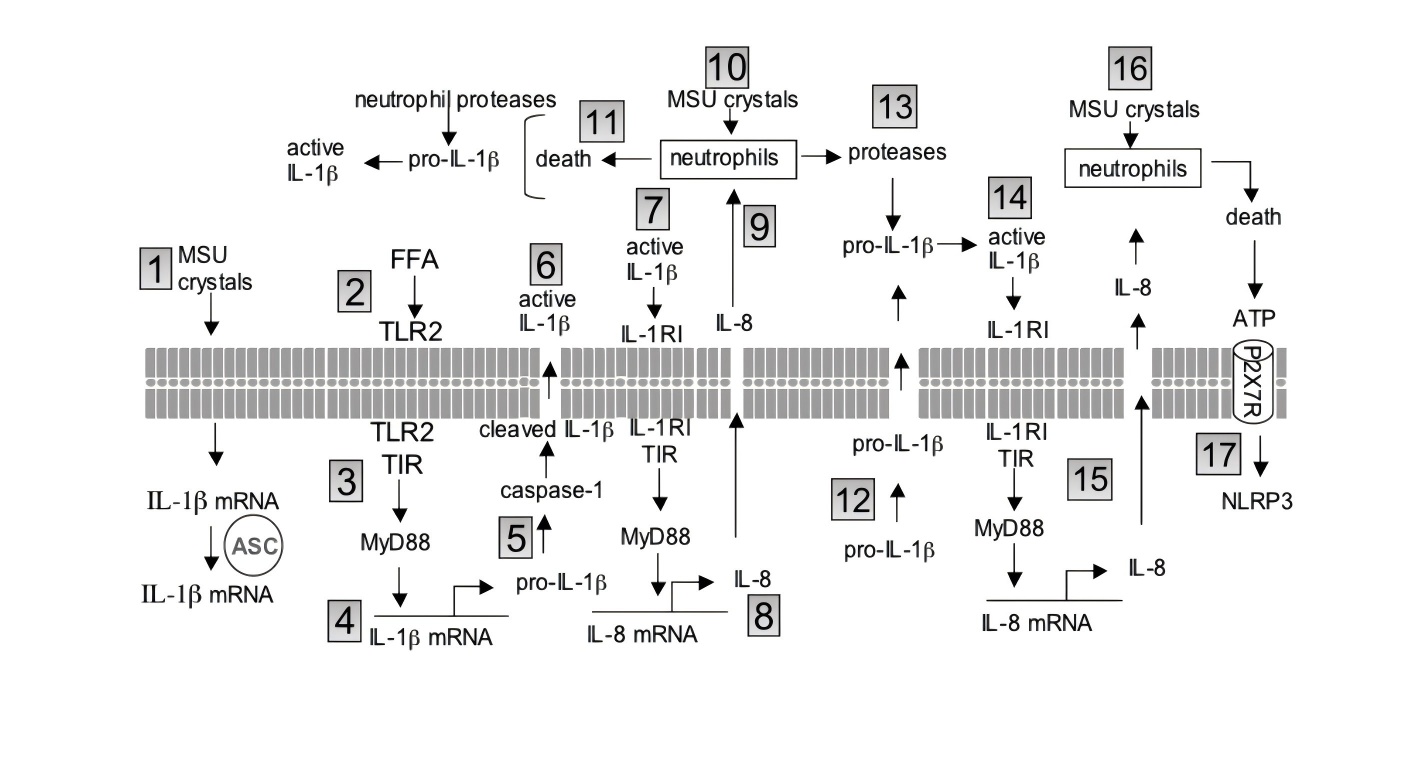
② Translation + Release
MSU crystals alone are insufficient to complete IL-1β protein translation; a “second signal” is required. TNF-α provides precisely this role: it induces neutrophils to express pro-IL-1β mRNA and activates the NLRP3-ASC-caspase-1 platform, cleaving and releasing mature IL-1β [1].
③ Positive Feedback Amplification
IL-1β binds to IL-1R1 → activates MyD88 → NF-κB re-enters the nucleus → further increases TNF-α; at the same time, it stimulates endothelial cells to secrete IL-8, recruiting more neutrophils and forming a “cytokine storm” loop [1].
III. Data : Key Findings from 2021–2025
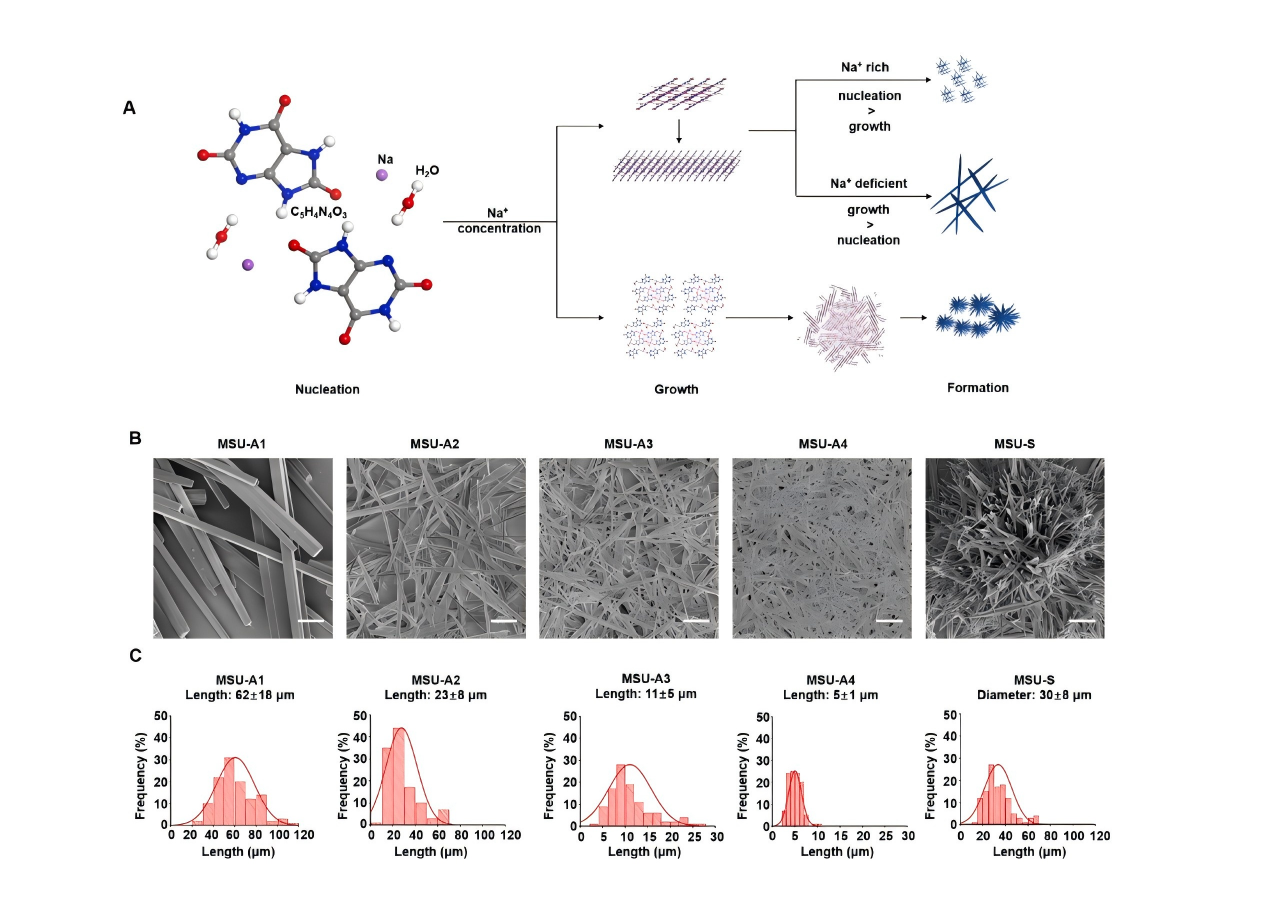
Chen et al. systematically revealed that the NLRP3 inflammasome and mitochondrial ROS are key drivers of MSU-induced inflammation. The physicochemical properties of MSU crystals play a pivotal role in acute gout, with medium-sized, high-aspect-ratio needle-shaped crystals being the most pro-inflammatory. They further proposed two potential therapeutic strategies—cholesterol clearance and antioxidant approaches—offering new insights into gout intervention [3].
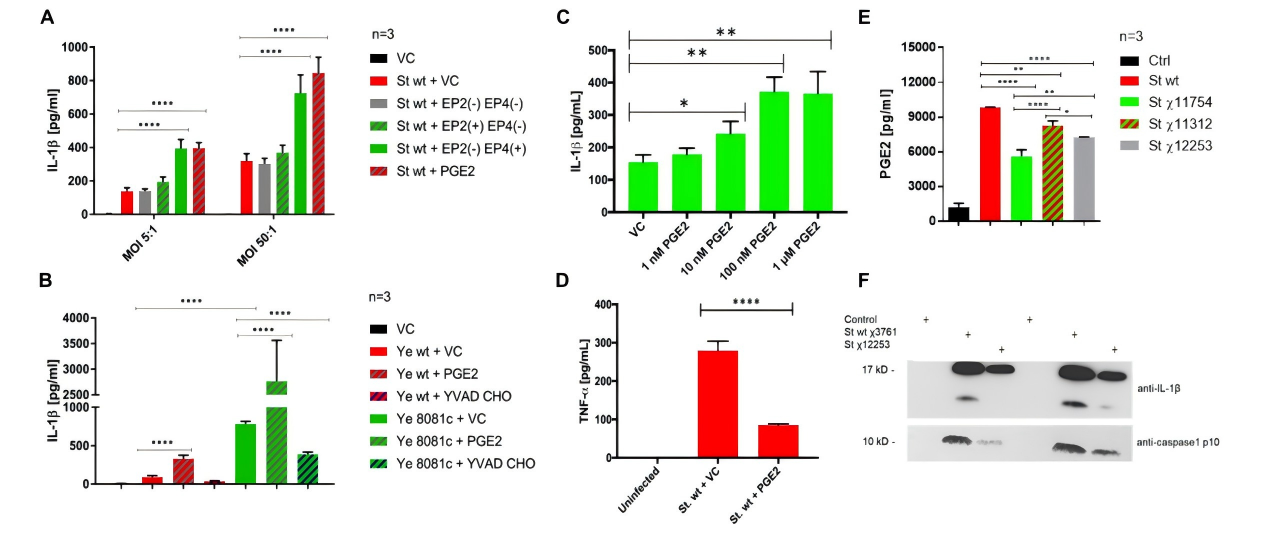
Sheppe et al. demonstrated that PGE₂ activates the inflammasome via the EP4 receptor, forming a positive feedback loop: inflammation → PGE₂ → IL-1β → more inflammation [4]. This provides theoretical support for developing immunoregulatory anti-infection strategies.
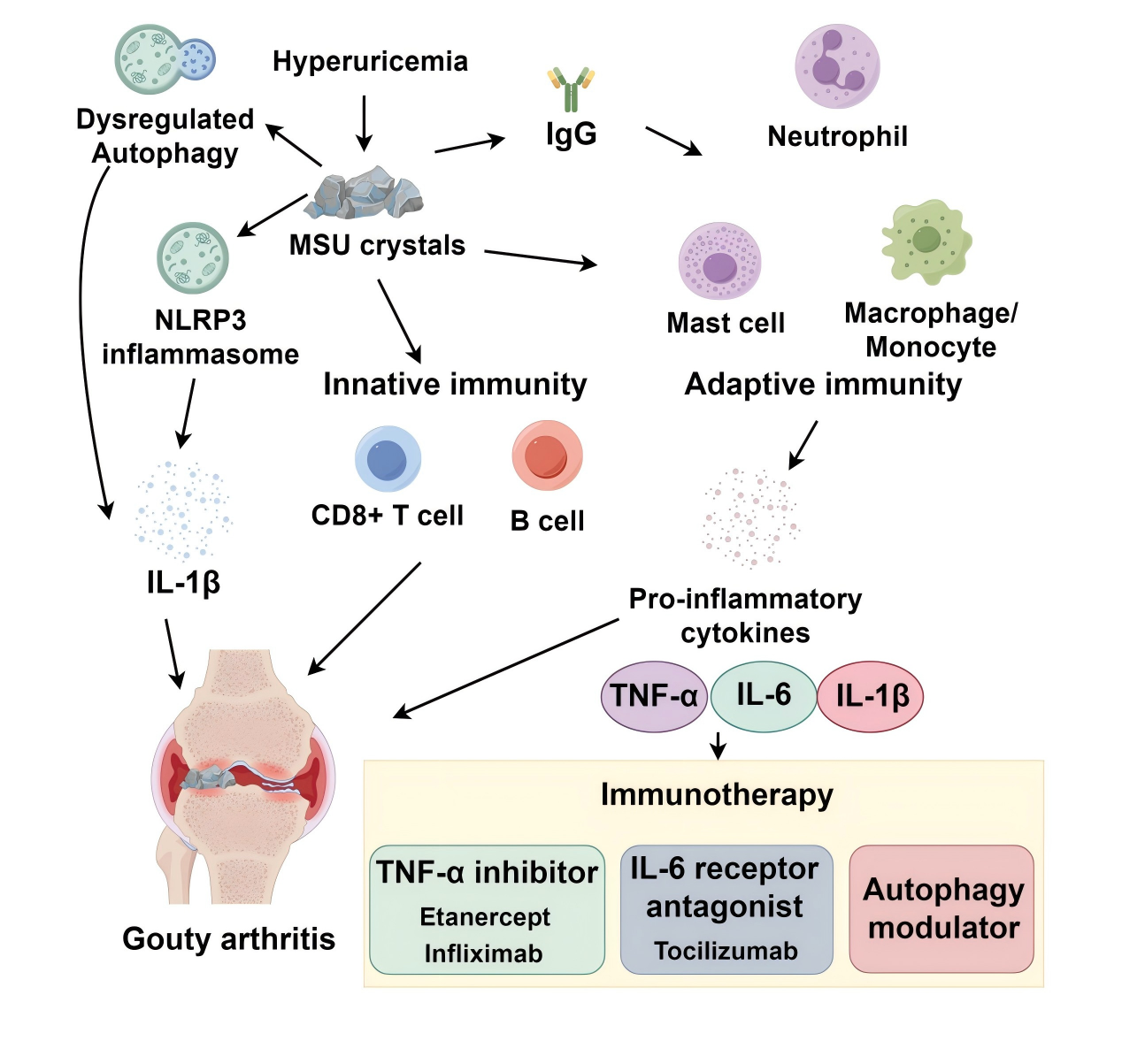
Chen et al., from a systems immunology perspective, explored the pathogenesis of gouty arthritis (GA), focusing on the role of immune responses and advances in precision-targeted therapies. They highlighted that conventional drugs (e.g., colchicine, NSAIDs) show limited efficacy, while biologics (e.g., TNF-α inhibitors, IL-6 antagonists) offer new options for refractory GA. They also pointed out that autophagy modulation, microbiome interventions, and epigenetic regulation represent promising directions for future precision therapies [5].
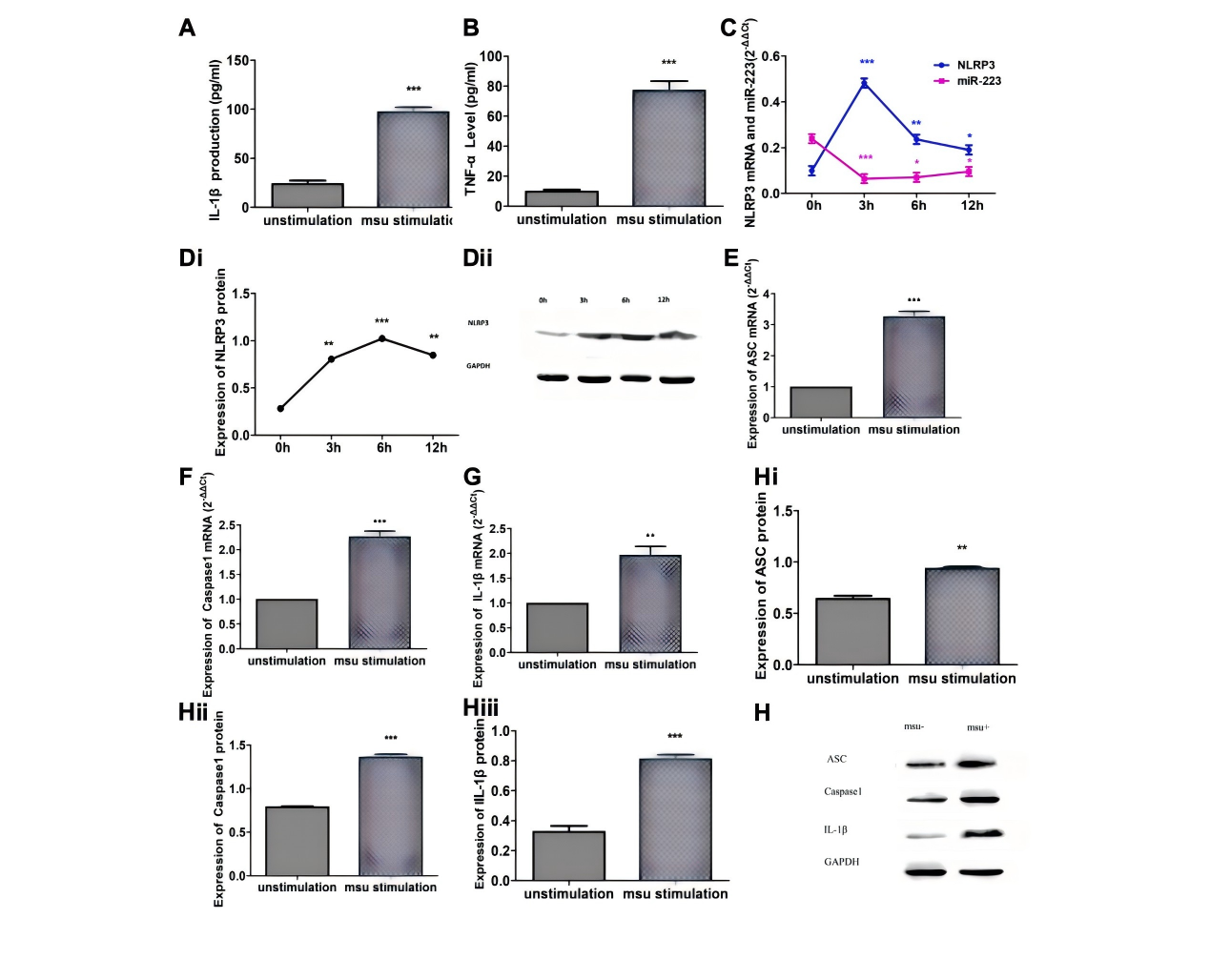
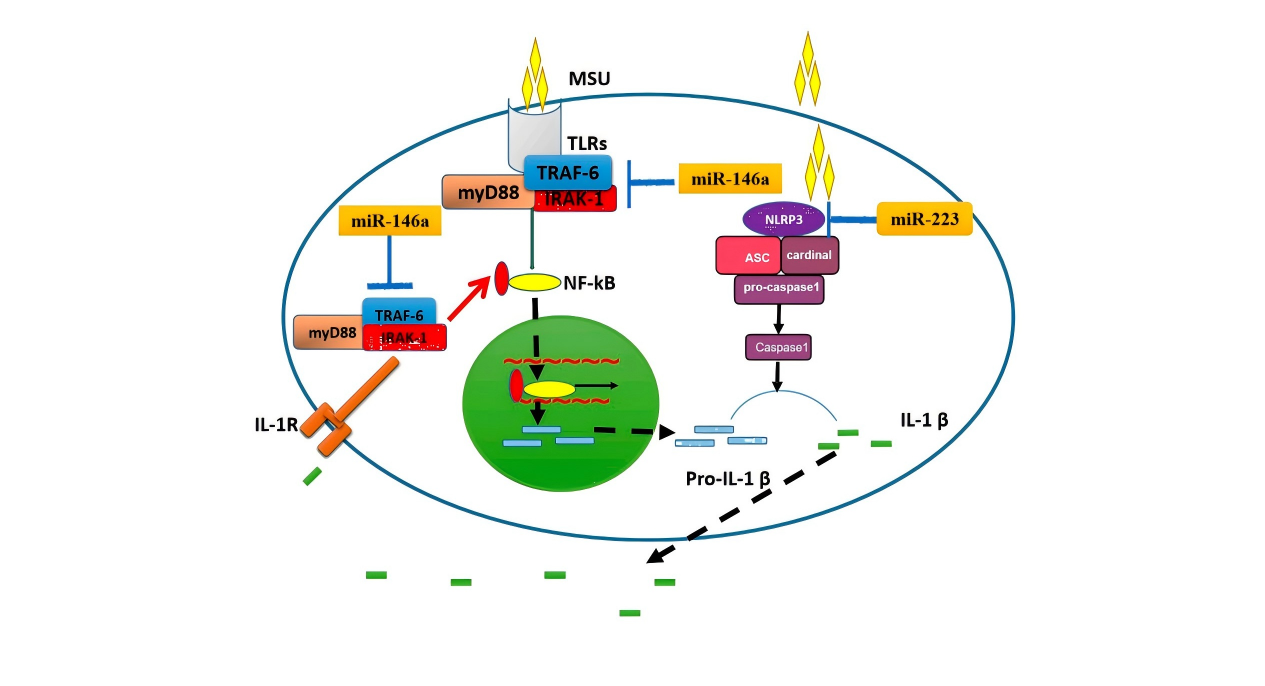
Zhang et al. established an inflammatory model by stimulating RAW264.7 murine macrophages with MSU crystals and, for the first time, systematically elucidated the negative regulatory role of miR-223 in gout inflammation. They showed that miR-223 targets the NLRP3 inflammasome, inhibiting the production of IL-1β and TNF-α, thereby contributing to the spontaneous resolution of acute gout flares. This suggests a novel therapeutic target—modulating miR-223 expression to control inflammatory responses [6].
IV. Clinical Implications: From Biomarkers to Dual-Target Intervention
An elevated IL-1β/TNF-α ratio indicates an impending flare, with combined measurement providing higher predictive accuracy than single biomarkers (AUC 0.87 vs. 0.79).
Therapeutic strategies:
Within the IL-1 pathway, Anakinra and Canakinumab are already approved, achieving up to 70% pain relief within 24 hours.
Within the TNF-α pathway, small-sample trials with Etanercept showed efficacy in refractory gout, with good safety profiles.
Combination strategies—IL-1 inhibition for rapid pain relief followed by TNF-α inhibition to reduce recurrence—reduced flare rates from 70% to 15% in animal studies (p < 0.01).
Baricitinib (a JAK inhibitor) and Tocilizumab (an IL-6R antibody) downregulate both TNF-α and IL-1β transcription, offering a dual-benefit approach for patients with coexisting RA and gout.
In an era of rapid advances in artificial intelligence and biomedical technologies—when AI can identify crystals in 3 seconds, when nanobodies can instantly neutralize IL-1β, and when gut microbiota formulations can “digest” purines for you—gout therapy is evolving from mere pain relief toward the possibility of cure.
Reference:
[1] Schett, Georg et al. “Why does the gout attack stop? A roadmap for the immune pathogenesis of gout.” RMD open vol. 1,Suppl 1 e000046. 15 Aug. 2015, doi:10.1136/rmdopen-2015-000046
[2]Dinarello, Charles A. “How interleukin-1β induces gouty arthritis.” Arthritis and rheumatism vol. 62,11 (2010): 3140-4. doi:10.1002/art.27663
[3] Chen, Chen et al. “Monosodium urate crystals with controlled shape and aspect ratio for elucidating the pathological progress of acute gout.” Biomaterials advances vol. 139 (2022): 213005. doi:10.1016/j.bioadv.2022.213005
[4] Sheppe, Austin E F et al. “PGE2 Augments Inflammasome Activation and M1 Polarization in Macrophages Infected With Salmonella Typhimurium and Yersinia enterocolitica.” Frontiers in microbiology vol. 9 2447. 31 Oct. 2018, doi:10.3389/fmicb.2018.02447
[5]Chen, Zilong et al. “A systems immunology perspective on gout pathogenesis and its precision-targeted treatment strategies.” Frontiers in immunology vol. 16 1615914. 19 Jun. 2025, doi:10.3389/fimmu.2025.1615914
[6] Zhang, Quan-Bo et al. “MicroRNA-223 Suppresses IL-1β and TNF-α Production in Gouty Inflammation by Targeting the NLRP3 Inflammasome.” Frontiers in pharmacology vol. 12 637415. 16 Apr. 2021, doi:10.3389/fphar.2021.637415
