Nature Sub-Journal New Discovery: Fat tissue harbors a "low-grade fever" that impairs insulin and slows down metabolism...
Introduction
If the human body is compared to a city, then adipose tissue is the "warehouse". We used to think obesity was simply due to "too many goods piled up in the warehouse" (fat accumulation). But the latest research from the University of Bonn School of Medicine, published in Cellular & Molecular Immunology, tells us:
"The warehouse is actually on fire, and it's a slow-burning low fire."
01.Low-Grade Chronic Inflammation – The Overlooked "Smoldering Fire" of Obesity
In traditional views, obesity is often simplistically attributed to an "energy imbalance." However, nearly 20 years of research has repeatedly shown that adipose tissue is far from a passive storage depot; it is an active endocrine-immune organ. Macrophages, T cells, and mast cells infiltrate it, secreting pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α, creating a state of chronic low-grade inflammation . When this inflammation persists, the insulin signaling pathway gets "scorched" by phosphorylation, glucose transporter GLUT4 struggles to reach the membrane, leading to insulin resistance and type 2 diabetes.
02.Human Data: IL-9 Negatively Correlates with the "Fire's Intensity"
The University of Bonn team focused their lens on IL-9 – an immune factor previously associated with "allergies."
A stratified study of 159 adults in Cameroon found: Serum IL-9 was significantly lower in diabetic patients not infected with worms. For every quartile increase in body fat, IL-9 decreased by about 15% (Figure 1). It was negatively correlated with CRP and body fat, and positively correlated with muscle mass and insulin sensitivity.
Thus, the authors proposed a hypothesis: IL-9 acts like a "fire extinguisher" in fat. Lose the extinguisher, and the fire rages.
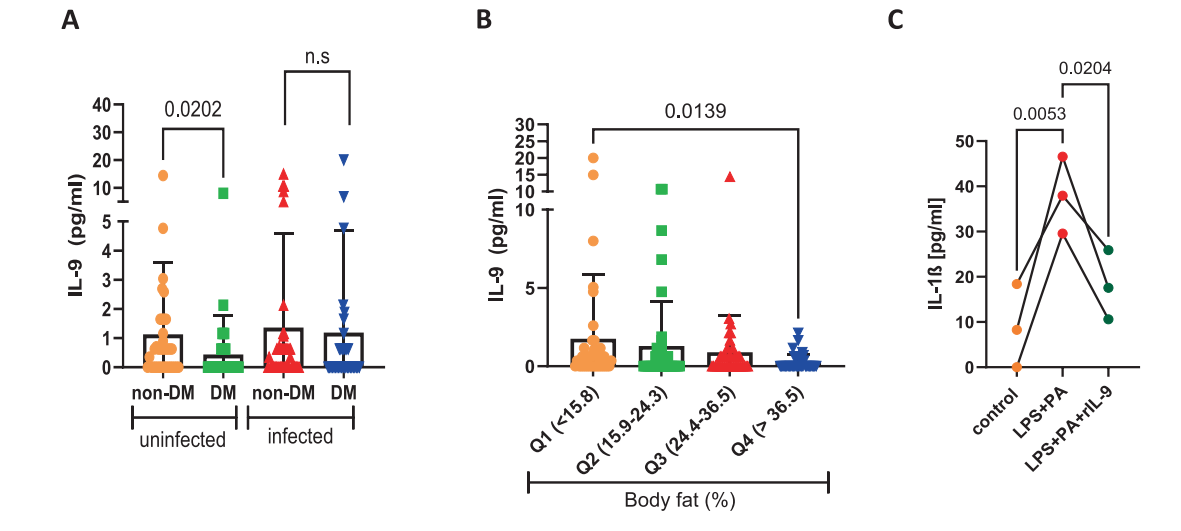
[Figure 1] The serum IL-9 level in patients with type 2 diabetes decreases.
03.Animal Model – Removing the "Fire Extinguisher", the Fire Rages Out of Control
Researchers experimented with IL-9 receptor knockout (IL-9RKO) mouse – effectively locking the fire extinguisher away. After 12 weeks on a high-fat diet (HFD): their weight increased by 20%, and the insulin tolerance test curve showed a "high plateau" (Figure 2). The proportion of CD11c+ macrophages in the fat pad doubled, while RELMα+ anti-inflammatory types decreased (Figure 3). Under acute cold stress, oxygen consumption dropped by 30%, and the expression of UCP1 and PGC-1α in brown fat was significantly reduced.
It appears that without IL-9, adipose tissue is in a state of "persistent low-grade fever," insulin signaling gets "fried," and even heat production goes on strike.
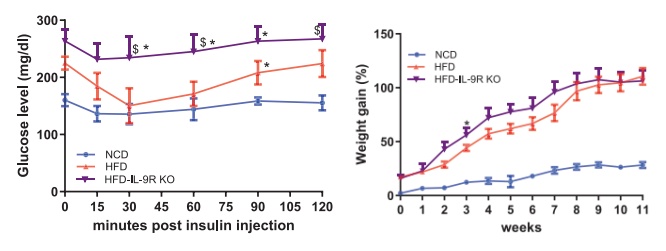
[Figure 2] IL-9R deficiency exacerbates metabolic deterioration during high-fat diet (HFD) feeding.
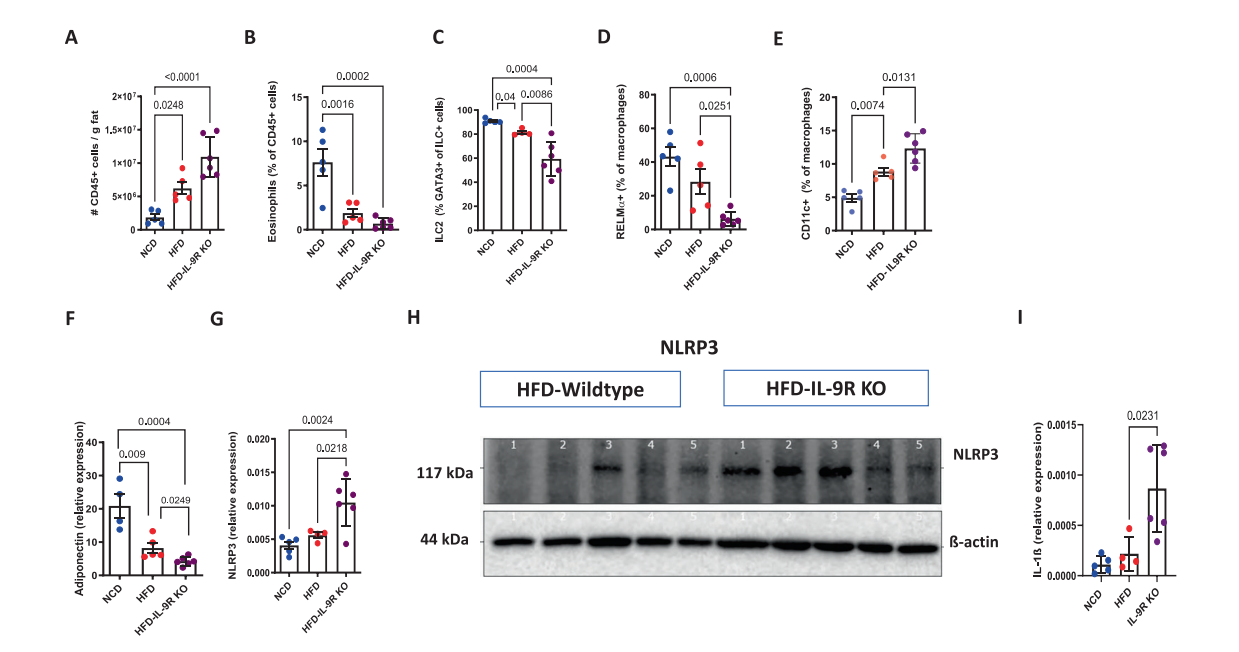
[Figure 3] IL-9R deficiency exacerbates inflammatory characteristics during diet-induced obesity.
A. Total number of immune cells per gram of adipose tissue; B. Eosinophils; C. Type 2 innate lymphoid cells (ILC2); D. RELMα⁺ macrophages; E. Frequency of CD11c⁺ macrophages;Adipose tissue; F. adiponectin; G. Expression of the NLRP3 gene; After 12 weeks of high-fat diet (HFD) feeding, the protein expression of; H. NLRP3 in the adipose tissue of wild-type and IL-9R knockout (KO) mouse, and the gene expression of IL-1β.
04.Locating the Ignition Source – The NLRP3 Inflammasome
The NLRP3 inflammasome is a multi-protein complex in the cytoplasm. Upon pattern recognition, it recruits ASC → Caspase-1, which cleaves pro-IL-1β/18, forms the pore-forming protein GSDMD, triggering pyroptosis (cell death) and the release of large amounts of inflammatory factors. Using in vitro cultures of bone marrow-derived macrophages (BMDMs) and adipose tissue macrophages, researchers found that NLRP3-mediated release of IL-1β and IL-18 was significantly reduced in normal mouse, but drastically increased in IL-9R deficient mouse. When IL-9R deficient mouse were injected with the NLRP3-specific inhibitor MCC950, the glucose tolerance AUC decreased by 35%, insulin sensitivity was restored, CD11c+ macrophages in fat decreased, and RELMα+ cells increased (Figure 4).
Thus, the ignition source is confirmed: NLRP3 = the igniter; IL-9 = the fire extinguisher; if the extinguisher is empty, the fire cannot be stopped.
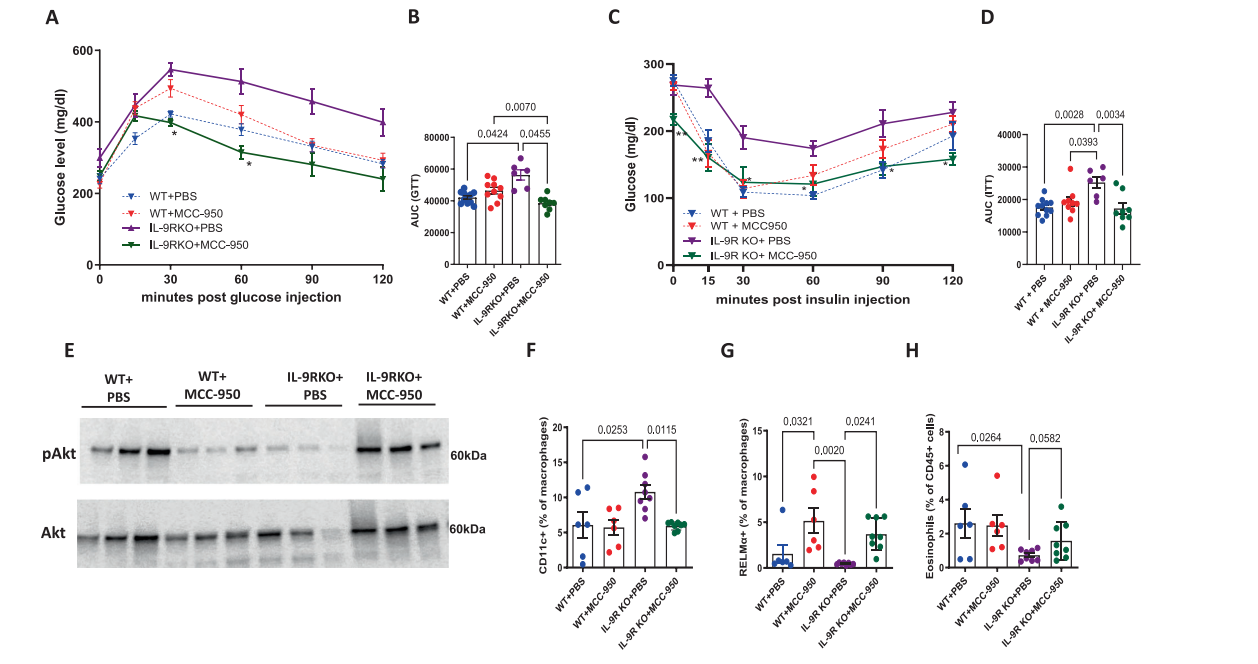
[Figure 4] Inhibition of NLRP3 signaling can improve the metabolic and inflammatory status of IL-9R knockout mouse.
05.Molecular Switch – STAT5 is Responsible for "Pumping the Water"
When researchers blocked STAT5 with an inhibitor, recombinant IL-9 (rIL-9) lost its ability to suppress NLRP3 and IL-1β. Furthermore, ChIP-qPCR showed that STAT5 can directly bind to the NLRP3 promoter region, negatively regulating its transcription (Figure 5). This, for the first time, writes the complete "IL-9 – STAT5 – NLRP3" axis into the map of metabolic inflammation.
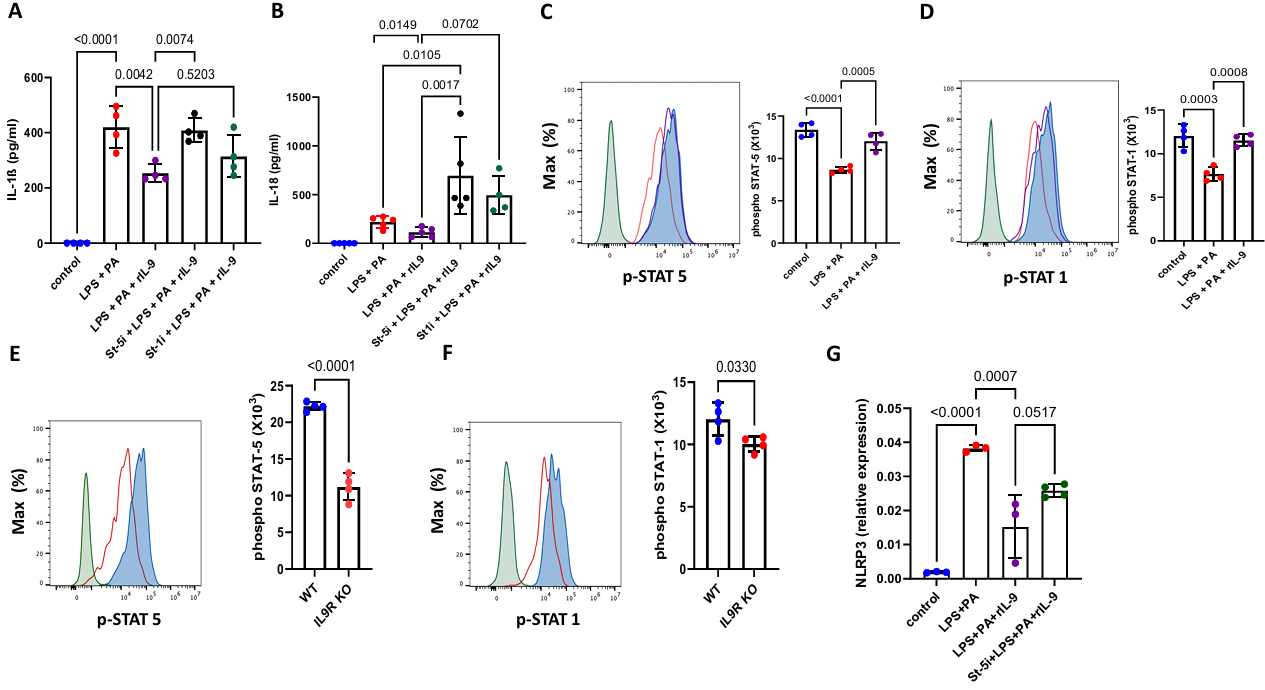
[Figure 5] IL-9 inhibits NLRP3 through STAT-5
06.The Worm's Revelation – Borrowing Worms for Training, Activating the Extinguisher
Researchers noted that in the African cohort, people infected with filarial worms had higher IL-9 levels. Therefore, they injected filarial worm antigen (LsAg) into obese mouse. After 30 days of infection, wild-type mouse showed improved insulin tolerance, and intrathoracic IL-9 levels increased threefold, whereas the protective effect was halved in IL-9R deficient mouse (Figure 6). This suggests that immune-modulating molecules derived from worms can indirectly "stoke" the IL-9 pathway, providing new ideas for the future development of "worm-inspired anti-inflammatory metabolic drugs."
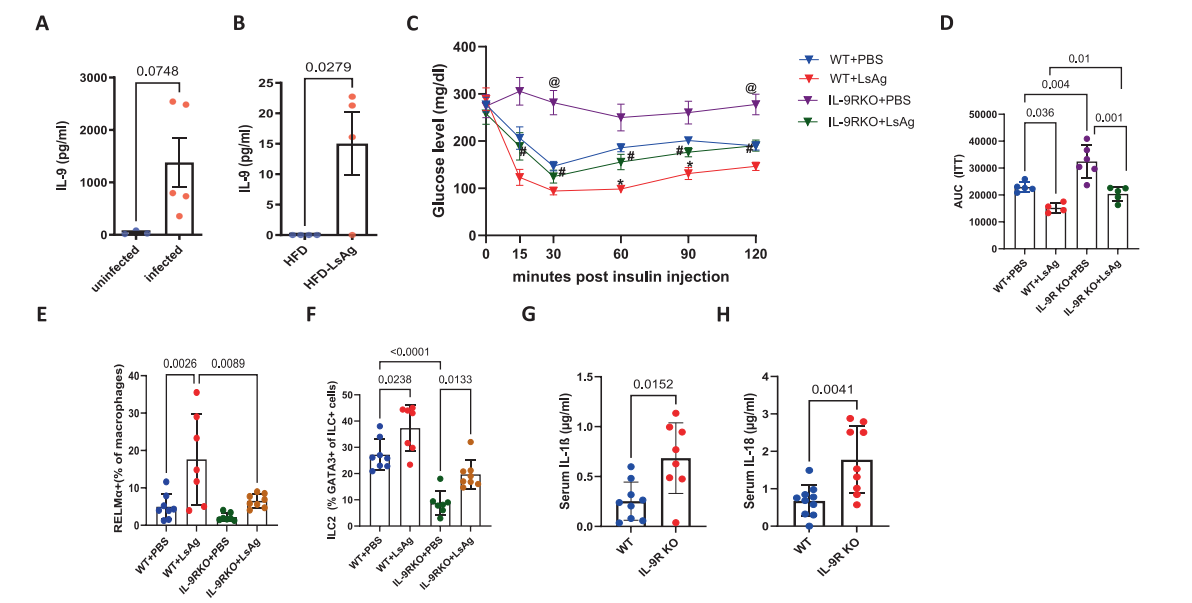
[Figure 6] The metabolic improvement effect of worm infection on diabetes is partially dependent on IL-9.
07.Conclusion – "Weight Loss" Might Require "Firefighting" First
Weight loss ≠ simply reducing "quantity," but also extinguishing the "fire." Chronic inflammation might be the true culprit behind plateaus, rebound weight gain, and insulin resistance.
Clinically, IL-9 could serve as a biomarker for early identification of "inflammatory obesity." Small molecules and biologics targeting the STAT5/NLRP3 axis are already being tested in autoimmune diseases; repurposing old drugs might be promising. IL-9 or related STAT5 agonists could become a new generation of "metabolic anti-inflammatory drugs." Furthermore, lifestyle factors like exercise, high-fiber diets, and sufficient sleep can all reduce NLRP3 activity. of course, don't "blindly eat worms"! Parasitic infection can carry other risks.
The flames in the "fat warehouse" may be small, but they are enough to burn down the entire "metabolic building." Next time you step on the scale, perhaps think:
Am I just fat, or am I "on fire"?
