In the laboratory, have you ever experienced such desperate moments? The carefully prepared samples suddenly become inactive, and the Western Blot (WB) bands vanish without a trace; the titer of repeatedly frozen-thawed samples plummets, and the ELISA test results are so low that you begin to doubt everything—even paler than your own face when the sample is loaded for color development; a slight tremor of the pipette directly "zeros out" the protein concentration... These "invisible assassins" are quietly devouring your proteins! Today, we will uncover the truth behind protein disappearance and find out where exactly your protein has "run off" to.
1. EP Tube Adsorption: The "Invisible Black Hole" for Proteins
Phenomenon: After protein purification or antibody aliquoting and storage, the concentration decreases inexplicably, and the experimental signals fluctuate unpredictably.
The truth lies in a material trap: the surface of some EP tubes (especially low-quality plastic tubes) retains hydrophobic groups, which can adsorb proteins. For low-concentration proteins (e.g., <10 μg/mL), the loss rate can be as high as over 30%. When the same detection antibody is aliquoted and stored in EP tubes from different manufacturers, the titer can differ by more than 5 times in ELISA experiments.
Solutions:
• Select low-adsorption, high-purity EP tubes (e.g., polypropylene ones). Before use, pre-block the tubes with 0.1% BSA or skimmed milk powder, or use these substances as protectants.
• Avoid storing proteins at low concentrations; try to keep the concentration of the protein or antibody to be stored above 0.1 mg/mL.
• When aliquoting proteins, avoid contact with the tube wall and gently mix the solution by pipetting with a pipette.
2. Repeated Freeze-Thaw Cycles: The "Lethal Cutting" of Ice Crystals
Phenomenon: Proteins and antibodies lose their activity after repeated freeze-thaw cycles, and "blank plates" appear in ELISA kits.
Structural Collapse: The formation of ice crystals during freeze-thaw cycles damages the three-dimensional structure of proteins, leading to aggregation or denaturation. Experiments show that after 5 repeated freeze-thaw cycles, the activity retention rate of antibodies or enzymes is less than 50%.
Shear Force Damage: The micro-shear force generated when ice crystals melt directly tears protein molecules.
High-Salt Damage: During freezing, the low-temperature environment and the reduced volume of the solution (e.g., in PBS) cause a locally high-salt environment in the solution, resulting in irreversible denaturation and inactivation of proteins and antibodies.
☑ Solutions:
• Aliquot for storage: Divide the sample into single-use aliquots (e.g., 50 μL), store them at -80°C for long-term use, and keep them at 4°C for short-term use.
• Add protectants: Glycerol (50%), trehalose, or BSA can reduce ice crystal damage; protease inhibitors can prevent protein degradation.
• Lyophilization protection: Sucrose, trehalose, and other substances in lyoprotectants act as excipients. After protein lyophilization, they provide a stable framework to maintain the spatial structure of protein products.
3. Pipette Operation Errors: A Tremor Ruins Everything
Phenomenon: The volume of sample added varies randomly, and the data of experimental groups and control groups are "unreasonably divergent".
Mechanical Damage: Uncalibrated pipettes, mismatched tips, or incorrect operating techniques (e.g., tilting the pipette tip) can increase the shear force on the protein solution, causing protein denaturation. Additionally, data shows that after one month of training, the coefficient of variation (CV) of experimental results for novice lab technicians can generally improve by more than 10%.
☑ Solutions:
• Calibrate pipettes regularly and use low-adsorption tips (e.g., silica gel tips).
• Practice frequently to avoid hand tremors.
• Centrifuge the protein solution (12,000 rpm for 3 minutes) before sample loading to prevent residue on the tube wall.
4. "Thrilling Moments" in Subpackage and Transportation
Phenomenon: The titers of aliquoted proteins vary greatly, and the proteins are completely ruined after transportation.
There are two main reasons for this situation: First, overly small aliquot volumes (e.g., <10 μL) are susceptible to evaporation and tube wall adsorption, leading to significant concentration deviations. Second, temperature fluctuations: repeated temperature changes during cold chain transportation (e.g., exposure to room temperature for more than 4 hours) accelerate protein degradation.
☑ Solutions:
•Ensure the aliquot volume is ≥10 μL and use pre-cooled EP tubes (-20°C).
•Add ice packs or dry ice during transportation and avoid exposure to room temperature as much as possible.
•Use lyophilized products for transportation.
5. Blind Faith in "Micro-Volume Kits" and "Rapid Kits"
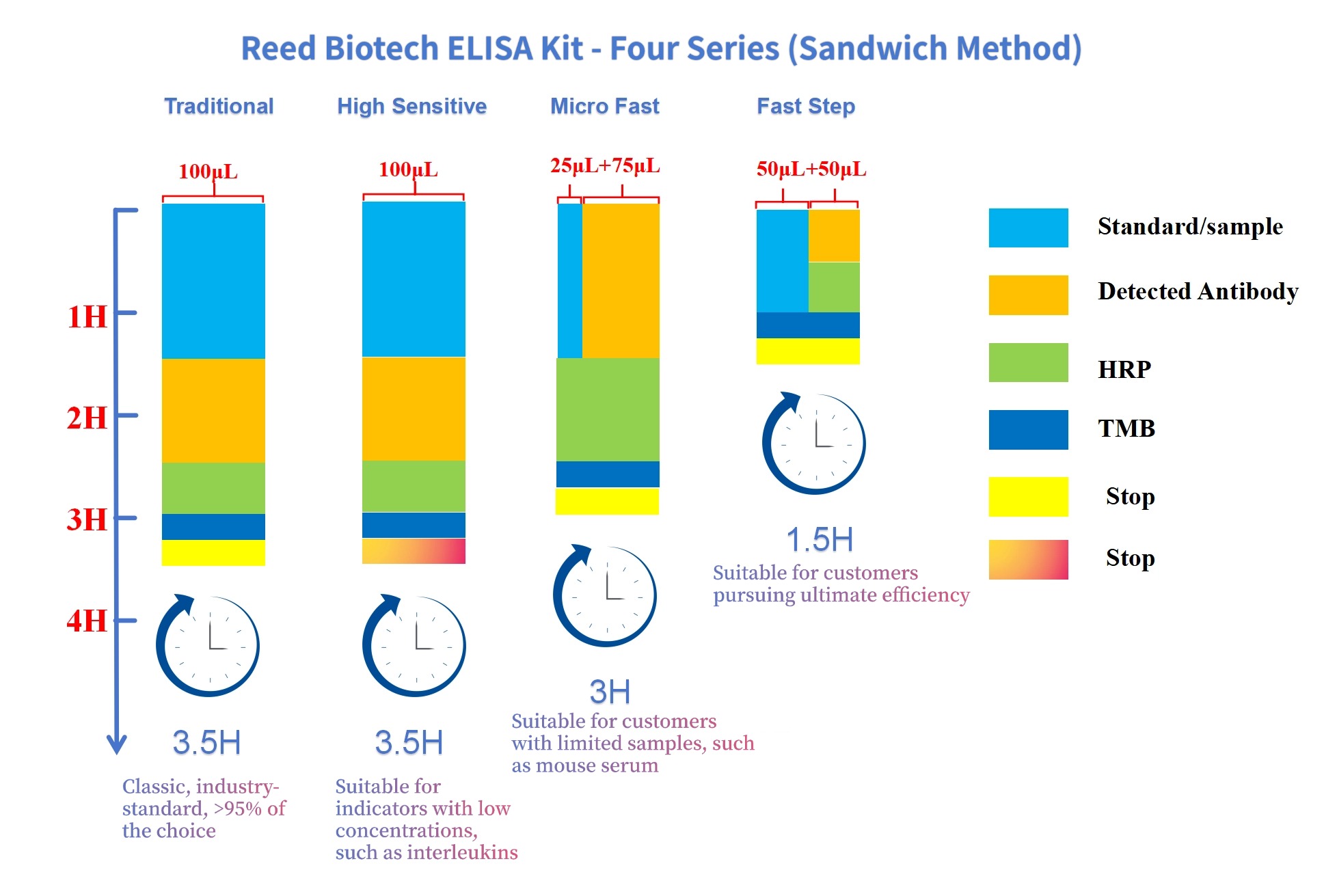
Some ELISA kit manufacturers offer a 50 μL micro-volume system (10 μL sample + 40 μL sample diluent), or claim that an ELISA experiment can be completed in just half an hour. However, such practices often lead to chaotic experimental trends and unreproducible results.
It should be noted that every reaction system has its own optimal volume and optimal reaction time. Sufficient quantitative changes are required to trigger qualitative changes, and the system cannot be simply scaled up or down proportionally. Take the most commonly used microtiter plates as an example: a 50 μL reaction system cannot even effectively wet the entire bottom of the microtiter plate well. Whether antigen-antibody reactions can proceed normally under such a system is questionable. Therefore, it is recommended that the minimum reaction volume be ≥100 μL.
☑ Solutions:
• Choose regular manufacturers with reasonable product designs, prepare a sufficient amount of samples, and use accurate pipettes.
Conclusion: Protect Proteins, Start with the Details
The standard substances and detection reagents of the ELISA kits from Reed Biotech adopt independently developed high-efficiency lyoprotectants and protein antibody protectants. All reagents have passed a 7-day stability test at 37°C (equivalent to 1 year of storage at 4°C). High-purity, low-adsorption EP tubes are used for aliquoting and storage to minimize adsorption and degradation. At the same time, a high-throughput automatic pipetting station has been introduced into the production process. Compared with manual production, this not only ensures the delivery time but also reduces the product CV to below 5%.