Introduction
Corneal neovascularization (CoNV) is a highly prevalent ocular disease that poses a severe threat to vision. Currently, anti-VEGF drugs (such as Ranibizumab) are commonly used in clinical practice, but they have drawbacks including short retention time in the eye, poor sustained efficacy, and the need for frequent injections. How can we extend the retention time of anti-VEGF drugs on the ocular surface and enhance their dual inhibitory effect on VEGF and its receptors, thereby improving the therapeutic outcome for corneal neovascularization?
01 Understand in One Sentence
A latest achievement by the team from Shandong First Medical University, published in Chemical Engineering Journal: The self-assembling peptide hydrogel NQ-Ran "neutralizes VEGF and blocks VEGFR", leading to approximately 70% regression of corneal neovascularization in mice within 7 days, and extending the drug retention time from 6 hours to 48 hours!

02 Research Highlights
•Intelligent Carrier: NapFF self-assembling nanofibers encapsulate Ranibizumab, forming a gel "drug reservoir" upon subconjunctival injection.
•Dual-Target Synergy: Ranibizumab (Ran) neutralizes VEGF, while the Q peptide occupies VEGFR, achieving a "two-pronged approach" to turn off vascular signals.
•Anti-Inflammatory Enhancement: Simultaneous downregulation of IL-1β, TNF-α, and IL-6, thoroughly eliminating the "flame" of vascular inflammation.
•Excellent Safety: No damage observed in the cornea, retina, heart, liver, spleen, lungs, or kidneys through H&E staining, indicating promising clinical translation potential.
03 Technical Route
① Peptide Synthesis
•Solid-phase synthesis of QKRKRKKSRYKS (Q) and NapFF-QKRKRKKSRYKS (NQ).
•Purity > 95% confirmed by HPLC, and molecular weight verified by ESI-MS.
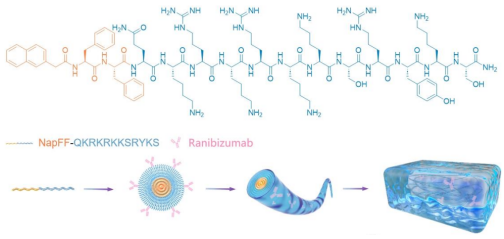
② Ranibizumab Conjugation
•Activation of carboxyl groups using EDC/NHS, followed by a 24-hour reaction with the -NH₂ group of NQ.
•Drug loading efficiency measured by BCA: 84.3% ± 0.8%.\
③ Self-Assembly into Gel
•After standing at room temperature for 5 minutes, NQ-Ran forms 10–15 nm nanofibers (observed via TEM).
•Rheometer results: G' (storage modulus) > G'' (loss modulus), confirming the gel state.
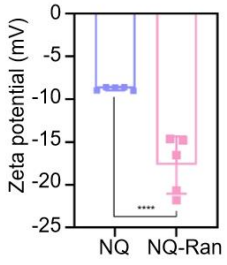
④ In Vivo Retention and Distribution
•Labeled with DiR, real-time imaging via IVIS showed that the fluorescence intensity on the ocular surface of the NQ-Ran group was still 6 times that of the free Ran group at 48 hours.
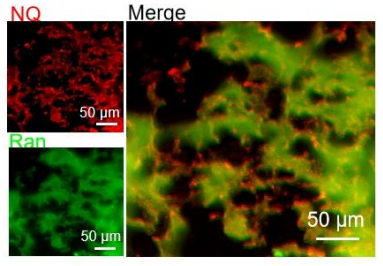
•Corneal flat-mount assay: Colocalization of NQ-Ran with CD31, clearly demonstrating vascular targeting.
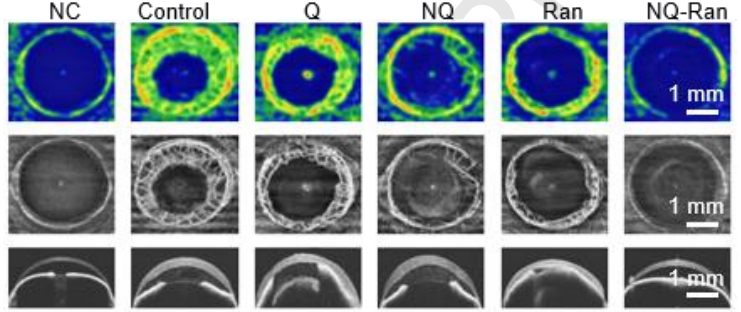
⑤ Efficacy and Mechanism
•Alkali-burned C57BL/6J mouse model, with 5 μL(240 μg/mL) administered via subconjunctival injection on Days 1/3/5.
•After 7 days, OCTA measurement of vascular area showed that the NQ-Ran group had only 22% of the vascular area compared to the PBS group (set as 100%).
•RNA-seq results: Downregulation of VEGFA, Flt1, and Icam1; upregulation of anti-inflammatory genes Fads2 and Diaph3.
•Protein chip analysis: TNF-α decreased by 10-fold, IL-10 increased by 3-fold.
04 Data Speaks
Neovascular area decreased by 78%.
Central corneal thickness decreased by 42%.
Drug retention time increased by 8-fold.
Inflammatory factor TNF-α decreased by 10-fold.
05 Clinical Significance
Alkali burns and post-corneal transplantation rejection are most feared for "abnormal blood vessel growth". This study encapsulates the clinically commonly used Ranibizumab into a self-gelling "sustained-release reservoir". A single subconjunctival injection allows the drug to remain on the ocular surface for 48 hours while blocking both VEGF and its receptors. If future optimization of dose and time-effect relationship can achieve "one injection lasting one month", it will not only reduce complications such as conjunctival hemorrhage and infection caused by multiple punctures but also provide a minimally invasive, long-acting, and scalable new strategy for "vascular regression" before corneal transplantation and "rejection prevention" after transplantation.
06 ReedBiotech – Providing Hardcore Data for This Study
The detection results of IL-1β, IL-6, and TNF-α in this study were all obtained using kits from ReedBiotech:
•High-sensitivity kits with pg-level sensitivity.
•Micro-volume kits requiring only 25 μL of sample.
•One-step kits enabling detection completion within 1.5 hours.
•Intra-batch variation < 6%, meeting the review requirements of SCI papers.
•Covering species such as humans, mice, and rats, fastest lead time.

|
Product Name |
Catalog Number |
Sensitivity |
Detection Range |
|
MF-Mouse IL-1β(Interleukin 1 Beta) ELISA Kit |
RE1074MF |
0.16 pg/mL |
0.78-50 pg/mL |
|
MF-Mouse IL-6(Interleukin 6) ELISA Kit |
RE3186MF |
4.69 pg/mL |
7.81-500 pg/mL |
|
MF-Mouse TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit |
RE1060MF |
6.56 pg/mL |
10.94-700 pg/mL |
|
Mouse VEGF-A(Vascular Endothelial Cell Growth Factor A) ELISA Kit |
RE1061M |
18.75 pg/mL |
31.25-2000 pg/mL |
|
MF-Mouse IL-10(Interleukin 10) ELISA Kit |
RE3187MF |
1.88 pg/mL |
3.13-200 pg/mL |
|
FS-Mouse MIγ/CXCL9(Monocyte Interferon Gamma Inducing Factor) ELISA Kit |
FS2783M |
1.42 pg/mL |
31.25-2000 pg/mL |
